UserWise University - Browse our elearning courses
Click here to learn more.
FDA Sponsored Research
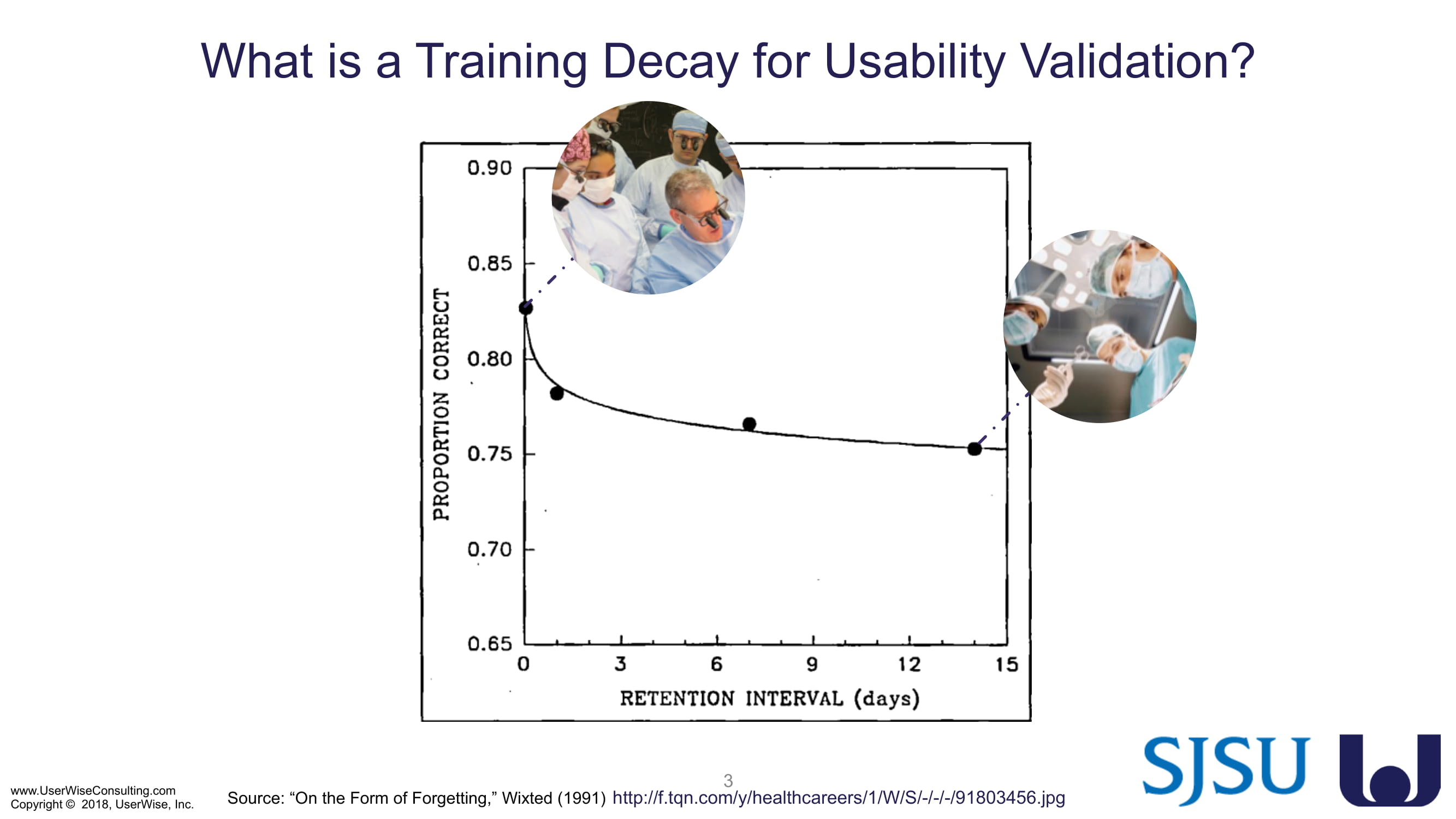
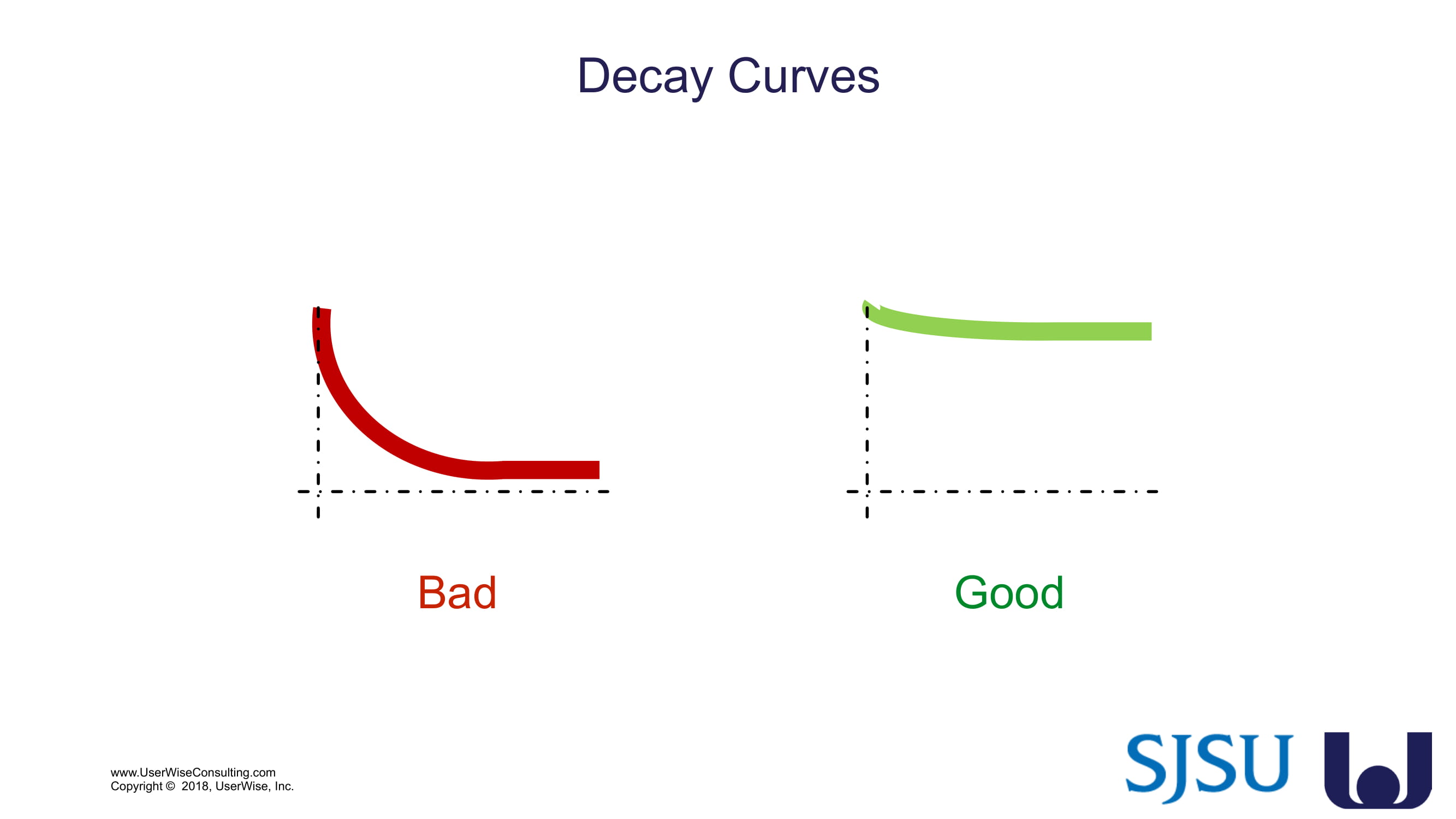
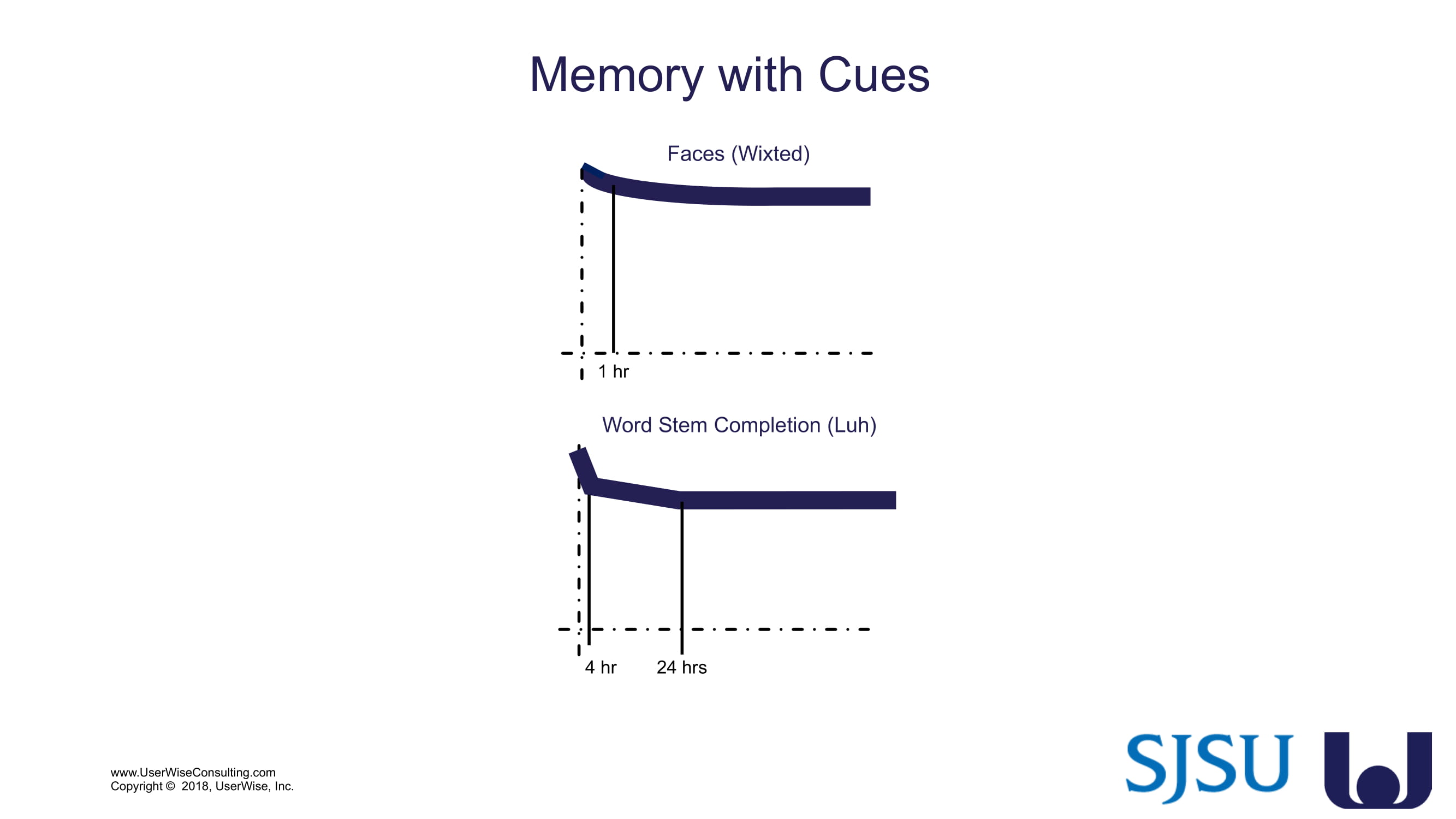
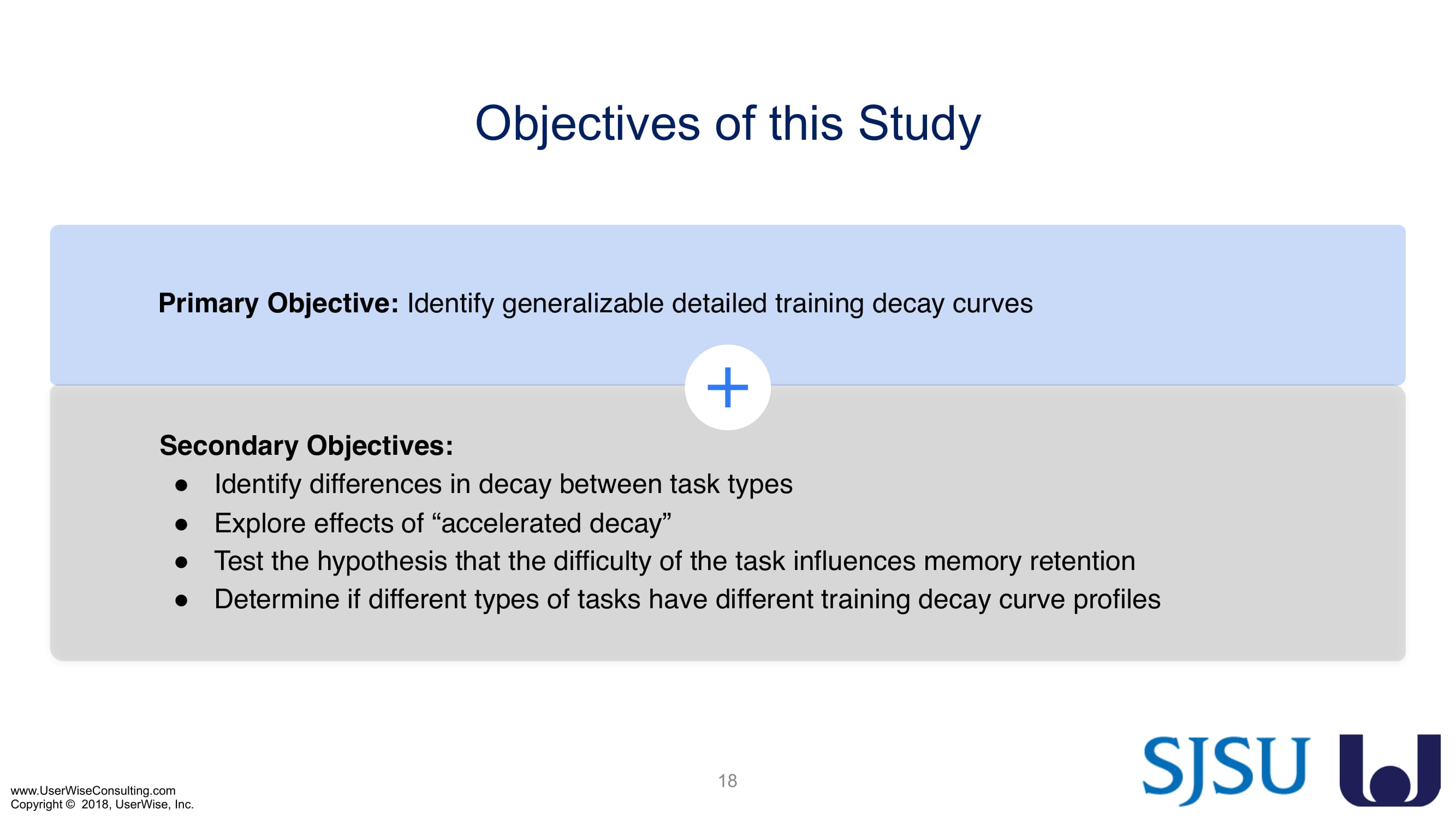
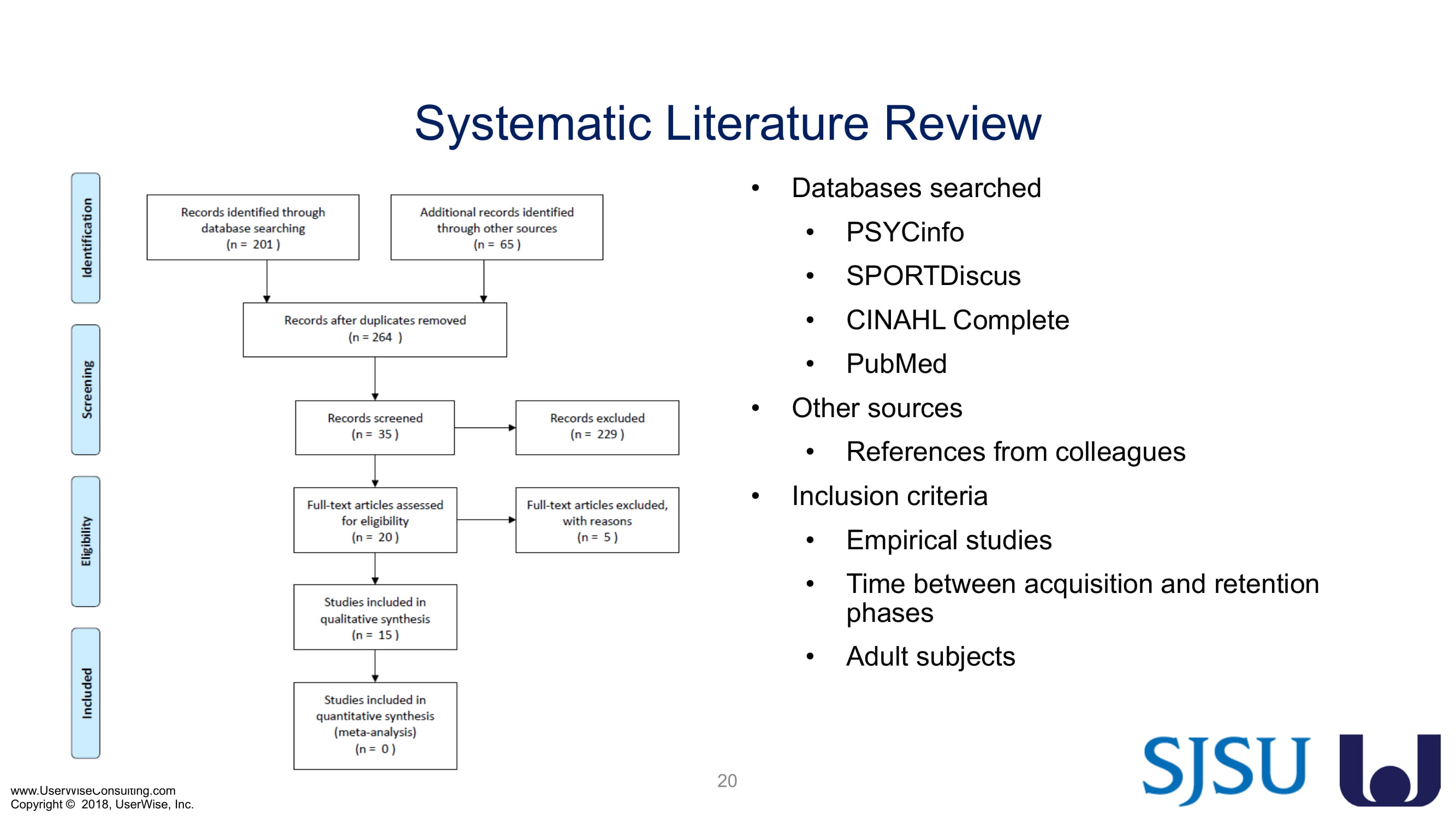
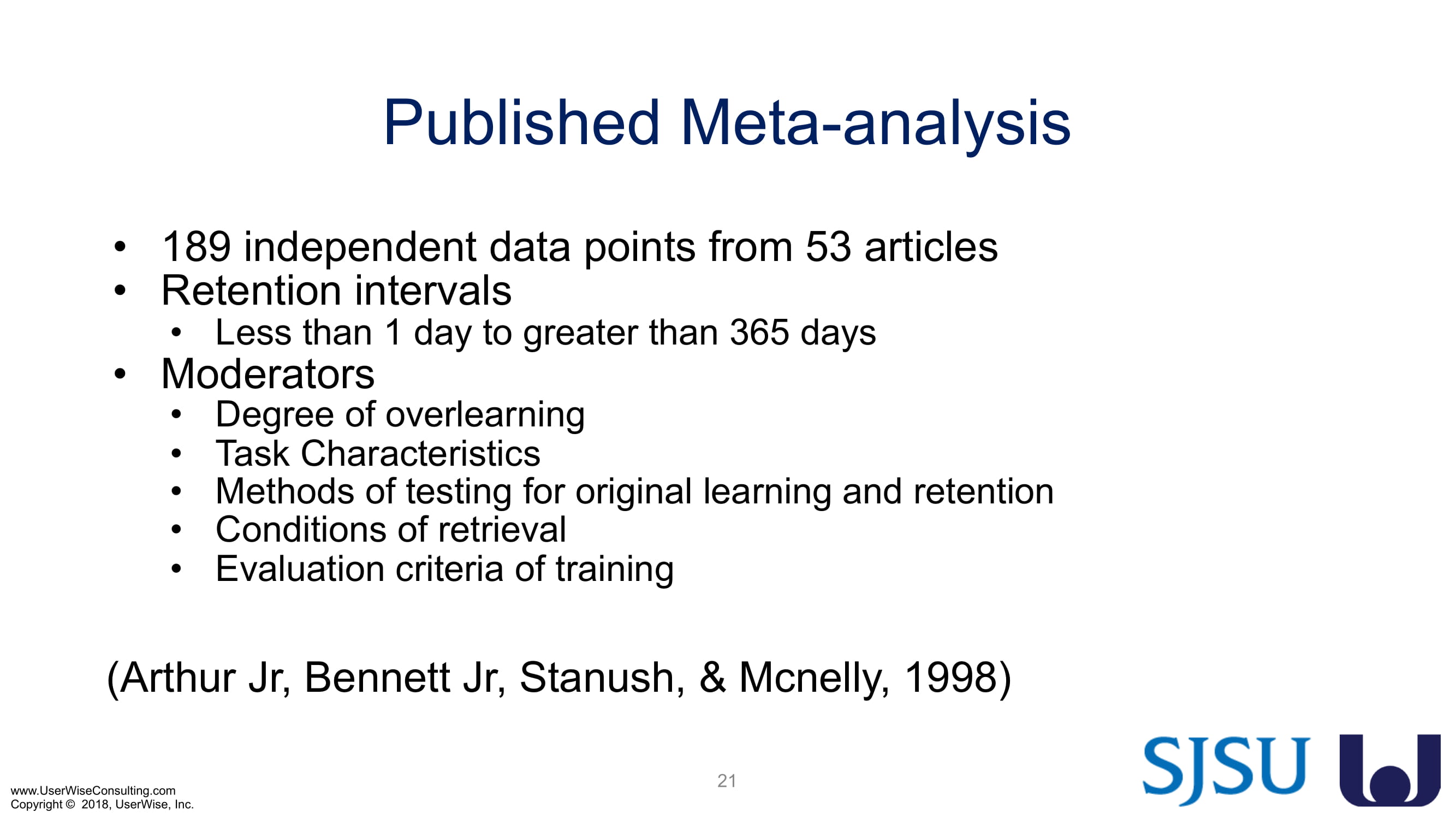
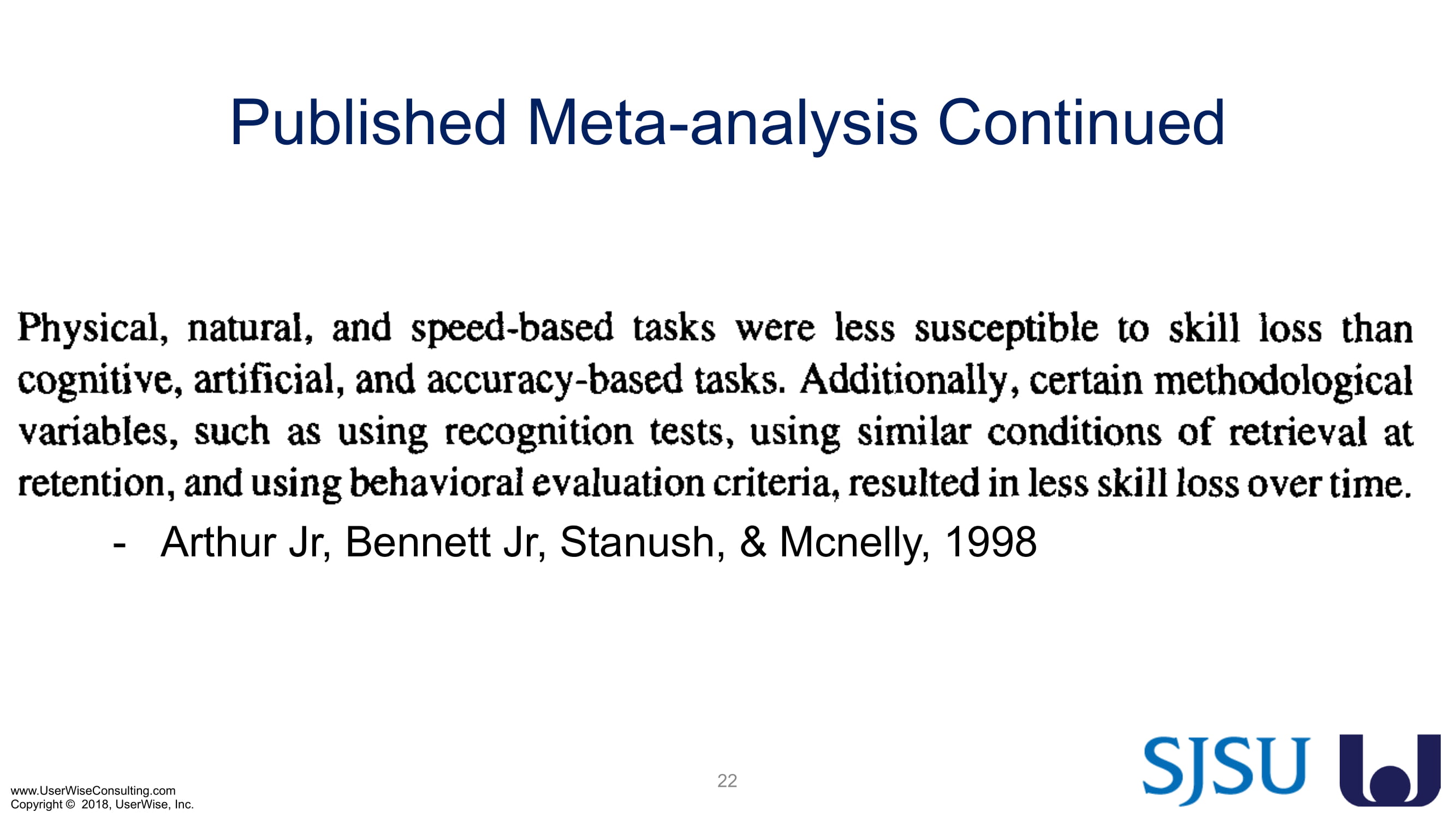
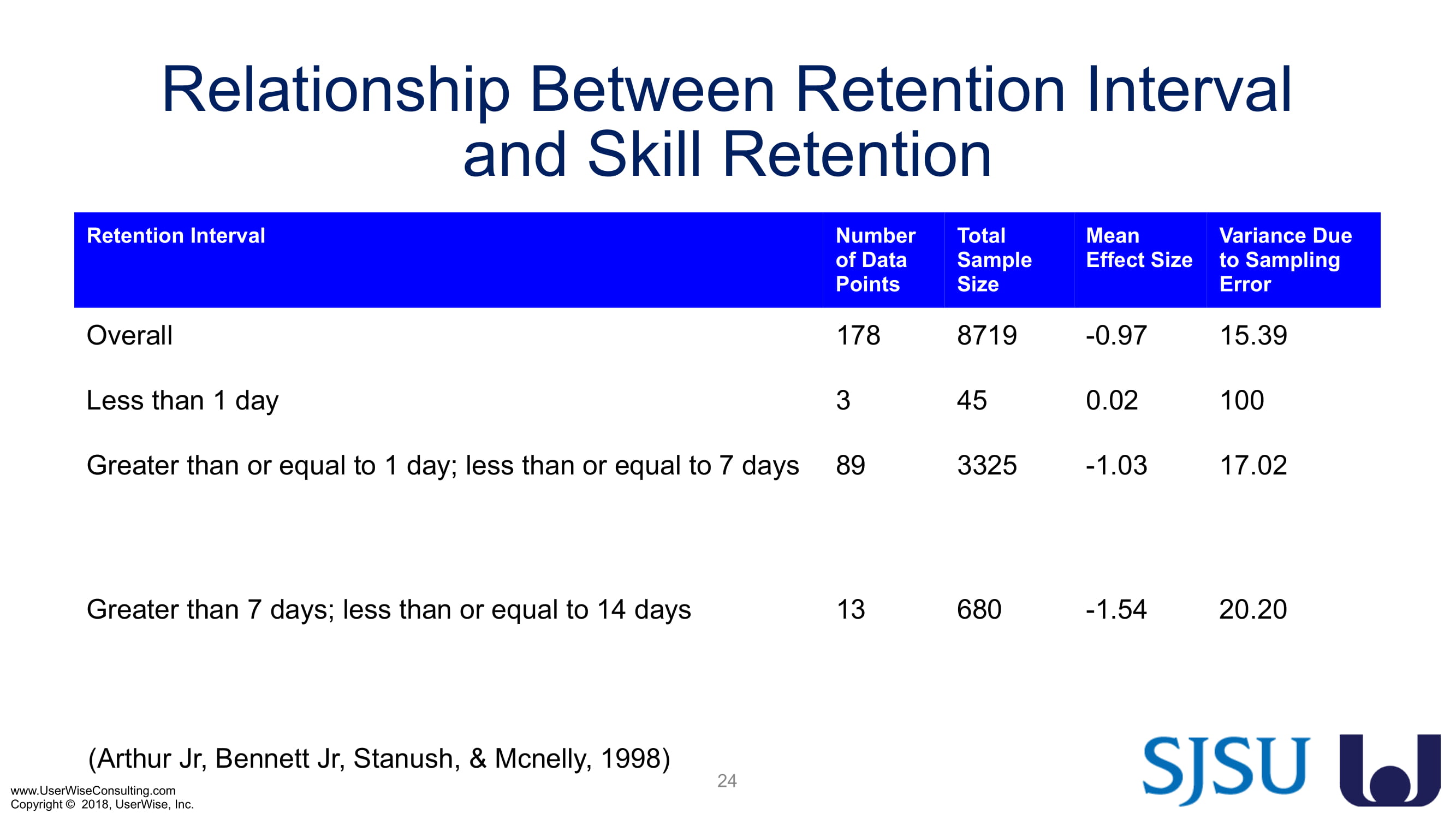
Training Decay Study
UserWise has been collaborating with the FDA and San Jose State University (SJSU) on FDA-grant-funded research to evaluate the impact of training decay duration on participant’s performance in medical product human factors validation testing.
In January 2020, we successfully completed an initial pilot study. In Q2 of 2022, we completed running sessions and are now excited to report on the "Training Decay Selection for Medical Product Usability Validation Testing”
results.
Subscribe to our Training Decay Newsletter and stay up-to-date on the grant research progress.
Background Information
The FDA invited UserWise, a ClariMed Company to present proposed training decay research at FDA headquarters in December 2017 to all of the FDA HF experts (e.g. Irene Chan, Shannon Hoste, Hannibey Wiyor, Xin Feng, Kimberly Konston, etc.). Earlier in 2017, Irene Chan encouraged UserWise to apply for a grant to further research training decay. The FDA then approved our White Paper, invited us to submit in the final stage of the grant proposal process, and formally accepted the grant application as a 2-year project.
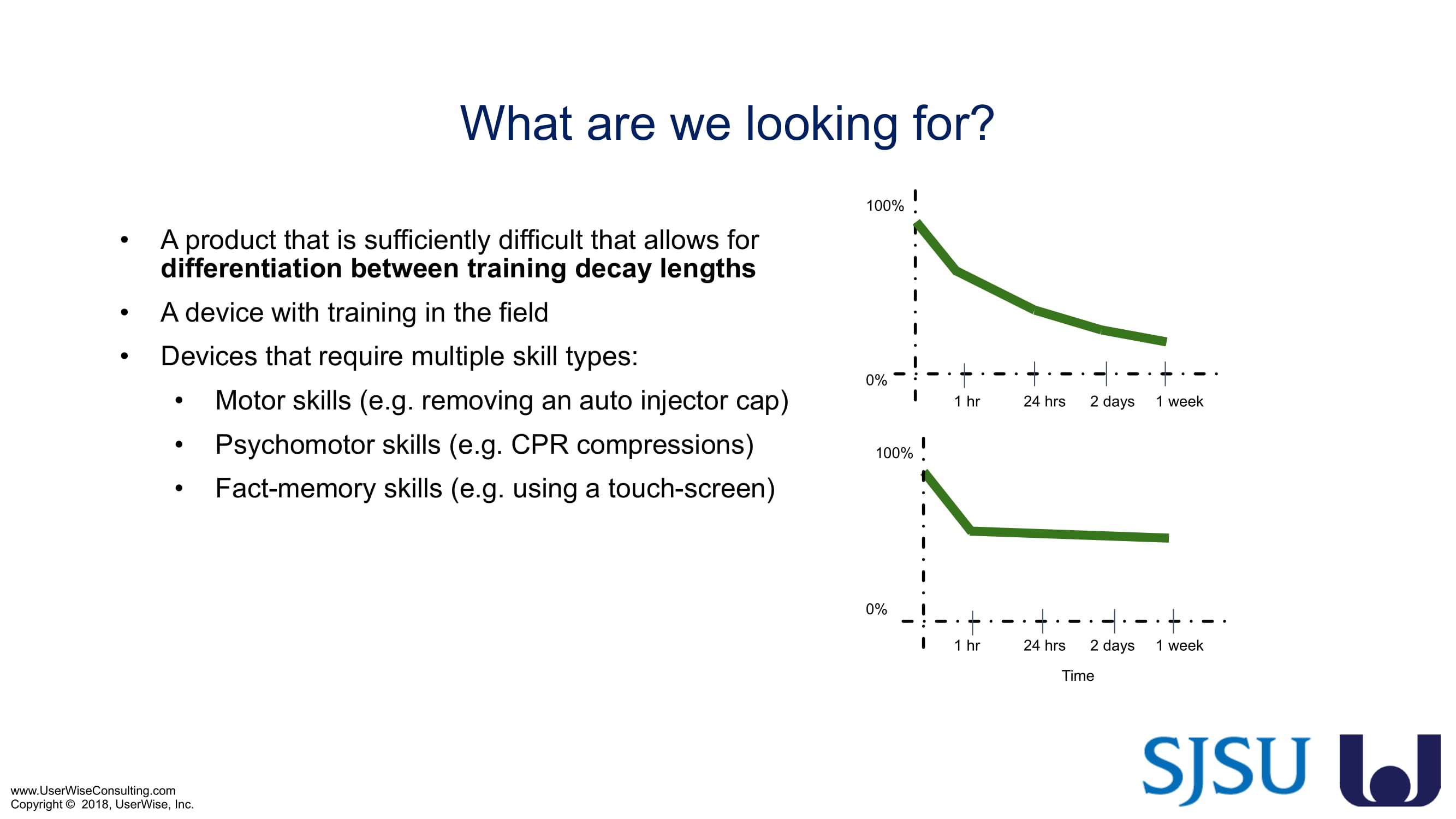
The research project, "Training Decay Selection for Medical Product Usability Validation Testing," which started in 2018, builds upon Clark’s paper, literature review, and presentation about training decay from 2016.
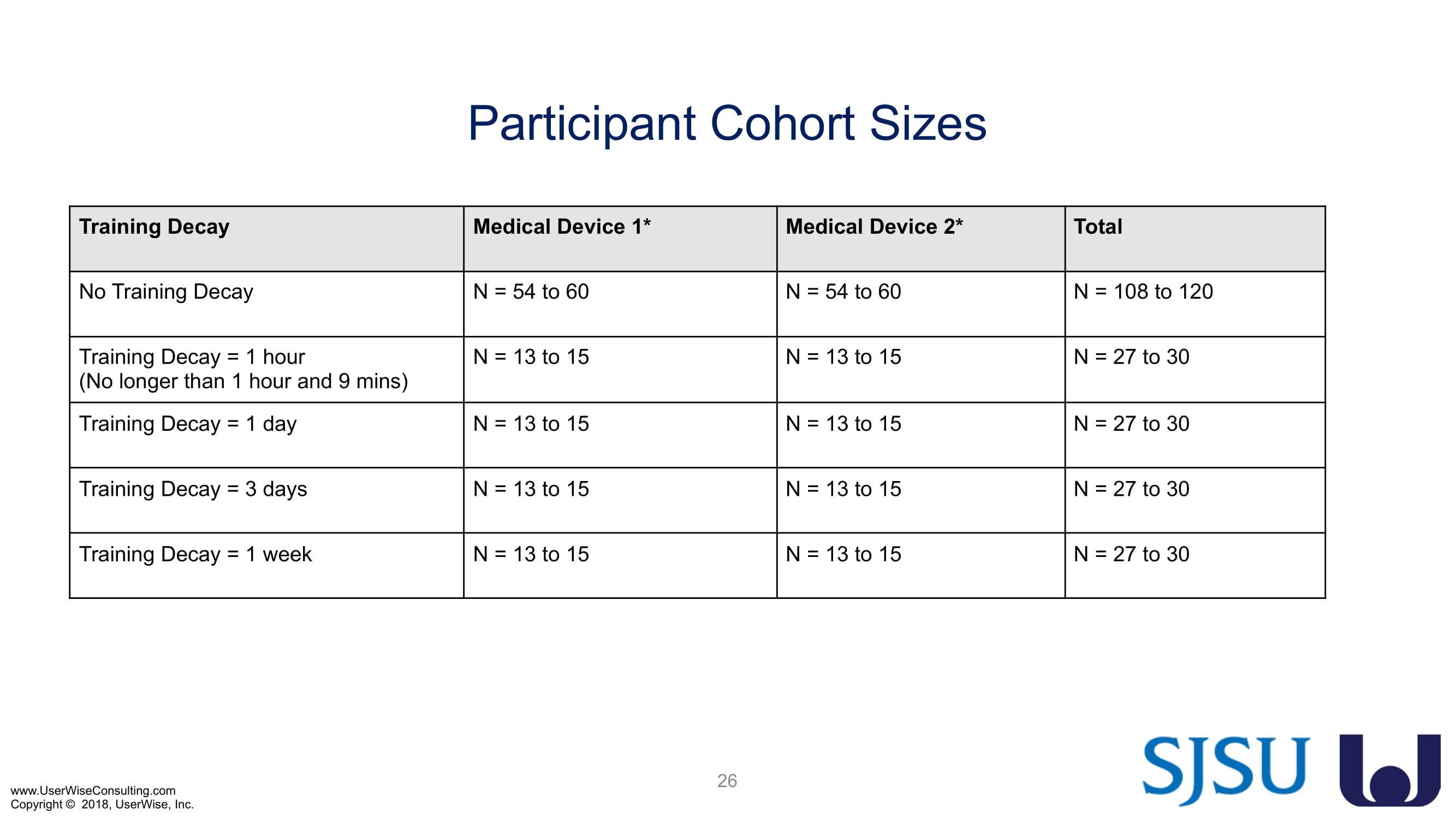
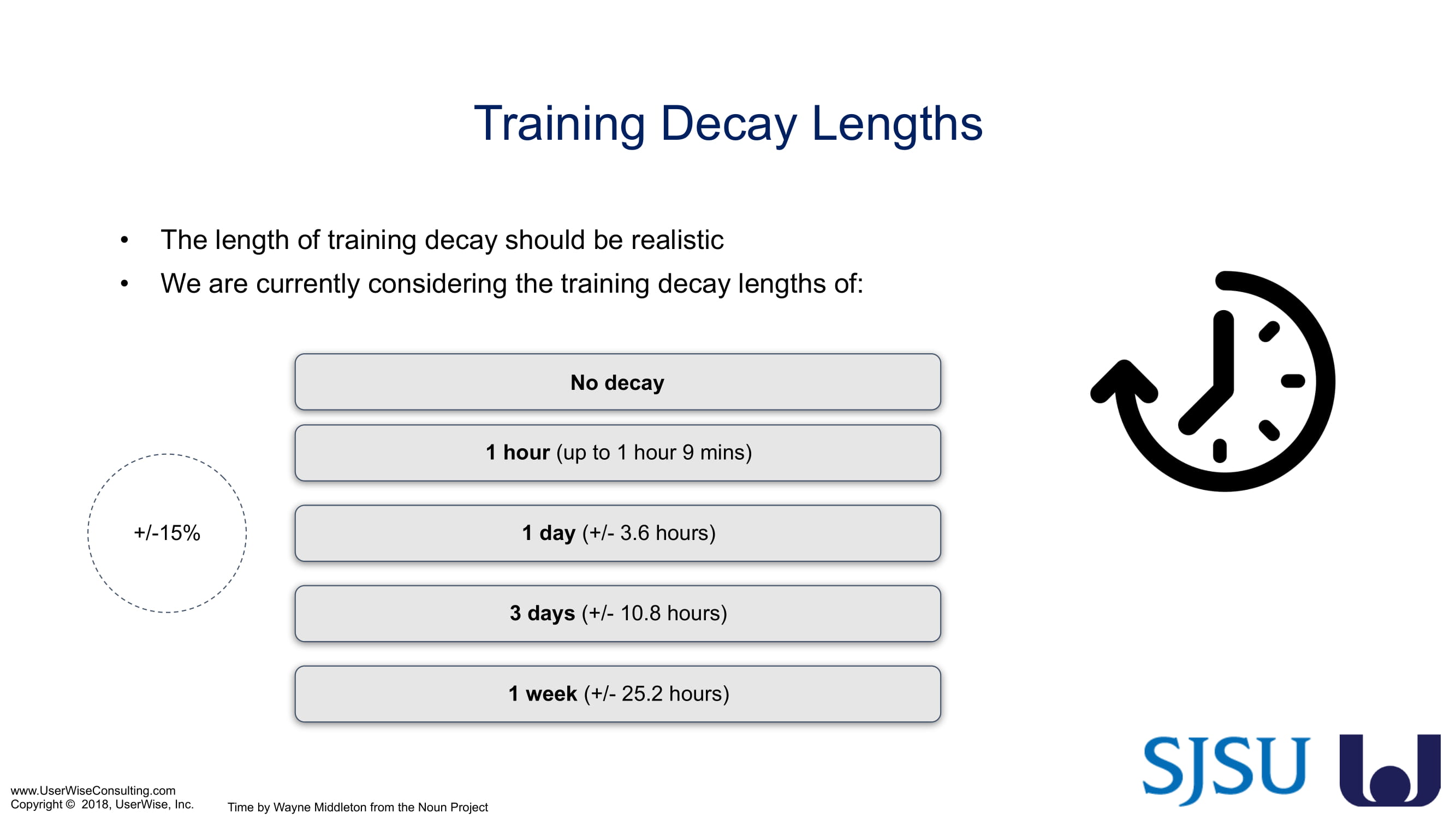
As of early 2020 we have selected an insulin device to use in the evaluation. The study design has been finalized to include training decay periods varying between no delay through a 7-day delay between training and testing. We are gearing up to complete testing later in 2020.
In Q1 of 2022 recruitment for Training Decay Sessions resumed. By the end of summer 2022, all sessions were concluded with the report set to be submitted for review at the start of fall.
December 2022 Update
UserWise is excited to announce that the study report submission has been received and is being reviewed by the U.S. Food and Drug Administration.